(Source – Keysight Technologies)
Australian medtech company to develop world first portable brain scanner
Medical technology or MedTech continues to be an area that sees constant innovation over the last few years. Compared to other industries, MedTech solutions are also becoming increasingly in demand as the healthcare industry continues to cope with an increasing number of patients globally.
Advancements such as AI and robotic solutions, as well as collaboration with medical technology companies and IT service companies providing cloud-based software and healthcare apps are likely to boost industry growth in the future.
When the COVID-19 pandemic disrupted lifestyles around the world, MedTech solutions were adopted by most hospitals globally to improve their patient care and also increase the speed of diagnostics. Apart from the pandemic, tech innovations have also witnessed some solutions being applied in numerous other medical procedures.
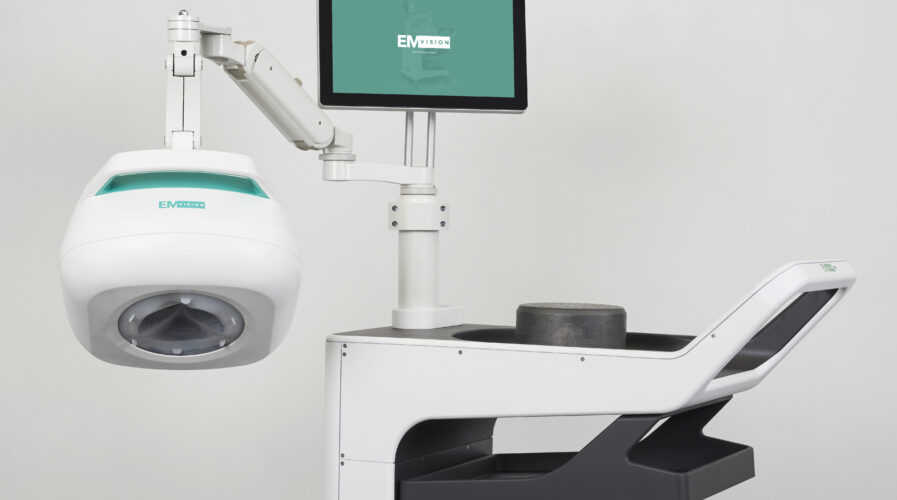
In Australia, EMVision has recently signed a strategic original equipment manufacturer (OEM) agreement with a US$26 billion global electronics test and measurement equipment supplier, Keysight Technologies. The Australian medical technology company is focused on the development and commercialization of portable medical imaging technology.
As a world leader in radiofrequency (RF) technology, the agreement signifies a strategic push into the MedTech sector for Keysight. It’s backing of EMVision’s breakthrough technology, affirms both the multi-billion dollar total addressable market for the scanners, as well as the global opportunity for Australian innovation.
With more than 445,000 Australians currently living with the effects of stroke, the relationship accelerates EMVision’s pathway to delivering faster diagnosis and treatment decisions within critical timeframes, improving global patient outcomes for stroke.
Under the agreement, EMVision is granted exclusivity over the supply of Keysight’s “fast sweep” Vector Network Analysers (VNA), which are core to the sensors used inside EMVision’s portable brain scanner. This VNA enables the EMVision device to be miniaturized, making it smaller and more mobile for environments, like Intensive Care Units or ambulances, where space is limited.
Alongside the Keysight agreement, EMVision is progressing In-hospital (1st Gen) and Pre-hospital (2nd Gen) device development, and preparing for its upcoming expanded, multi-center clinical trials across NSW, VIC, and QLD. These studies are intended to support future regulatory clearances, including TGA, CE, and FDA, to begin commercializing EMVision’s game-changing product. The breakthrough technology, which is on track to go commercial in the coming years, has the potential to improve survival numbers and limit the long-term impact of stroke.
According to Dr. Ron Weinberger, CEO and Managing Director at EMVision, the integration of the Keysight VNA accelerates their pathway to commercialization, allowing them to bring a best-in-class portable imaging solution one step closer for patients and clinicians.
“Keysight’s support of our mission to deliver a faster, more accessible diagnostic tool promises great benefit to stroke patients around the world, and I look forward to the commercial phase of our relationship,” commented Dr. Weinberger.
At the same time, Australia has witnessed an increasing number of MedTech companies recently. In fact, revenue in the MedTech market is projected to reach US$523 billion in 2022, with the largest segment being medical devices.
READ MORE
- The criticality of endpoint management in cybersecurity and operations
- Ethical AI: The renewed importance of safeguarding data and customer privacy in Generative AI applications
- How Japan balances AI-driven opportunities with cybersecurity needs
- Deploying SASE: Benchmarking your approach
- Insurance everywhere all at once: the digital transformation of the APAC insurance industry